AspireAssist PATHWAY Trial shows 37% excess weight loss

At 52 weeks, mean percent body weight loss at 52 weeks was12.1±9.6% (14.2±9.8% for completers only) in the AspireAssist group and 3.5±6.0% (4.9±7.0% for completers only) in the Lifestyle Counseling group
Aspire Bariatrics has announced the results of its Pivotal Aspiration Therapy with Adjusted Lifestyle (PATHWAY) study of AspireAssist, a system that consists of a low-profile implantable gastrostomy tube and a siphon system, AspreAssist treatment was associated with greater weight loss than lifestyle counseling alone. Using the AspireAssist System, patients drain the contents of their stomachs after a meal, reducing caloric absorption by approximately 30%. The AspireAssist is given in conjunction with lifestyle therapy, in which patients are taught portion control, careful chewing, and other healthy lifestyle habits.
“The PATHWAY study showed that AspireAssist procedure is a viable alternative to bariatric surgery for patients with Class II or Class III obesity, with both significant weight loss and excellent safety. Side effects such as nausea and vomiting are rare, and, notably, the treatment group showed improvement in eating habits, with better food choices and more thorough chewing. This is partly due to the procedure mandating follow-up with the healthcare team for ongoing use, or the device shuts off,” said Dr Christopher Thompson, Associate Professor of Medicine at Harvard Medical School, Director of Therapeutic Endoscopy at Brigham and Women’s Hospital, and co-Principal Investigator of the PATHWAY Study.
According to the company, the AspireAssist provides a novel approach to obesity treatment through portion control. It is intended for long-term duration of use, and is to be used in conjunction with diet and exercise counselling and medical monitoring. The device is implanted in a 15-minute outpatient procedure, is fully reversible, and does not alter the patient’s internal anatomy. The AspireAssist received premarket approval from the FDA in June 2016 and is available commercially in the US, Europe, Australia, and New Zealand, and is indicated for obese patients, with a BMI35-55, who are age 22 or older and have failed to achieve weight loss using nonsurgical means. It's intended to be used for long-term weight loss and maintenance for a year or two or longer. The device is not to be used on patients with eating disorders.
The device requires a surgeon to insert a tube in the stomach with an endoscope via a small incision in the abdomen. It connects to a disk-shaped port that lies on the skin of the abdomen. About 20 to 30 minutes after a meal, the patient attaches an external connector and tubing to the valve, which opens to drain the stomach contents. The process takes about five to 10 minutes. It has an automatic shut-off feature that halts its functioning after 115 cycles of use; this requires the patient to make a physician visit to continue therapy and helps ensure that patients aren't misusing the device. There are a series of potentially risks associated with the placement of the tube, as well as the maintenance of the port valve. These include infection, leakage, bleeding, and persistent fistula development.
The results of the PATHWAY study, a 207 patient, multicenter randomised control trial in the US, were published in the paper, ‘Percutaneous Gastrostomy Device for the Treatment of Class II and Class III Obesity: Results of a Randomized Controlled Trial’, published in the American Journal of Gastroenterology.
A total of 207 participants were randomised in a 2:1 fashion: 137 to AspireAssist and 70 to Lifestyle Counseling and baseline characteristics were not different between the groups. Among those randomised, 26 AspireAssist and ten Lifestyle Counseling participants withdrew before beginning the study so 111 in the AspireAssist group and 60 in the Lifestyle Counseling group enrolled in the study (N=171). In total, 82 AspireAssist (74% of those enrolled) and 31 Lifestyle Counseling participants (52% of those enrolled) completed the entire 52-week study. The co-primary end points were mean percent excess weight loss and the proportion of participants who achieved at least a 25% excess weight loss. Participants in both treatment groups were seen by the study team for medical monitoring, lifestyle counseling, and blood tests at weeks 0, 2, 6, 10, 14, 20, 24, 28, 32, 36, 40, 44, 48, and 52. An assessment of eating behaviors (assessed by the Questionnaire on Eating and Weight Patterns-Revised and the Eating Disorder Examination) was made at baseline and at weeks 28 and 52, in both treatment groups, and an additional assessment at week 14 in the AspireAssist group.
Outcomes
At 52 weeks, mean percent body weight loss at 52 weeks was12.1±9.6% (14.2±9.8% for completers only) in the AspireAssist group and 3.5±6.0% (4.9±7.0% for completers only) in the Lifestyle Counseling group (Figure 1a-d). The difference in the mean percent body weight loss between the two groups was 8.6% (95% CI: 6.2–10.9). A greater proportion of participants in the AspireAssist group than in the Lifestyle Counseling group lost 10% or more of their initial body weight (58.6% vs. 22.0% and 69.5% vs. 19.4% in the completers only analysis) (Figure 1b). Mean weight loss of the mITT population was 14.2±11.3 kg in the AspireAssist group and 4.1±7.2 kg in the Lifestyle Counseling group.
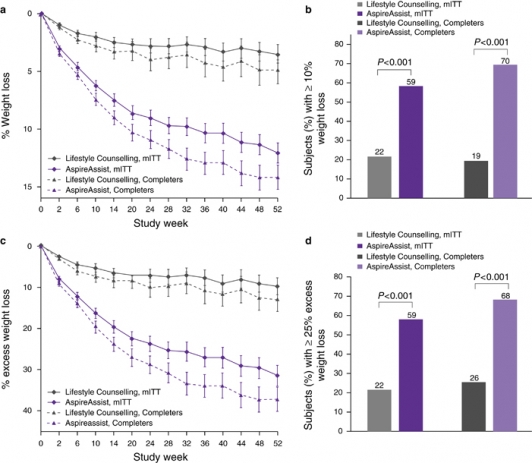
Figure 1a-d: Effect of AspireAssist on excess weight loss and percentage weight loss. Mean percentage body weight loss (a) at each study visit is shown, according to study group, for the modified intention-to-treat population (with multiple imputation for missing values) and for those who completed the entire study. The proportion of participants who lost 10% or more of their total body weight at 52 weeks (b) is shown for the modified intention-to-treat population and for those who completed 52 weeks. I bars (a,c) indicate s.e. Mean percent excess weight loss (c) at each study visit is shown, according to study group, for the modified intention-to-treat population (with multiple imputation for missing values) and for those who completed the entire study. The proportion of participants who lost 25% or more of their excess weight at 52 weeks (d) is shown for the modified intention-to-treat population and for those who completed 52 weeks.
Participants in the AspireAssist group had lost a mean (±s.d.) of 31.5±26.7% of their excess body weight (37.2±27.5% for completers only), whereas those in the Lifestyle Counseling group had lost a mean of 9.8±15.5% of their excess body weight (13.0±17.6% for completers only) (Figure 1c). The difference in %EWL achieved between groups was 21.7% (95% CI 15.3, 28.1), which was greater than the 10% threshold needed to achieve the a priori definition of success (p=0.008). A greater proportion of participants in the AspireAssist group than in the Lifestyle Counseling group lost at least 25% of their excess body weight (58.6% vs. 22.0% in a mITT analysis and 68.3% vs. 25.8% in a completers only analysis) (Figure 1d). Several sensitivity analyses confirmed the beneficial effect of AspireAssist in the co-primary end points.
For the AspireAssist group, at week 52 compared with baseline, clinically significant improvement was seen in HbA1C (−0.36% relative to 5.7% baseline, p<0.0001), triglycerides (−9.9%,p=0.02), and high-density lipoprotein cholesterol (+8.1%, p=0.0001), while modest improvement was seen in systolic blood pressure (−1.2%, p=0.38), diastolic blood pressure (−2.6%, p=0.06), low-density lipoprotein cholesterol (−4.2%, p=0.06), and total cholesterol (−2.5%, p=0.07). For the Lifestyle group, at week 52 compared with baseline, moderate improvement was seen in HbA1C (−0.22% relative to 5.7% baseline, p<0.0001), while modest or no improvement was seen in triglycerides (+0.1%, p=0.62), high-density lipoprotein cholesterol (+1.7%, p=0.55), systolic blood pressure (−2.5%, p=0.17), diastolic blood pressure (+0.5%, p=0.83), low-density lipoprotein cholesterol (−1.8%, p=0.72), and total cholesterol (−2.5%, p=0.28).
The differences in improvement from week 52 relative to baseline with the aforementioned parameters between the AspireAssist group and the Lifestyle Counseling group were not statistically significant, except for glycated hemoglobin. The Impact of Weight on Quality of Life score increased in both treatment groups, across all five measures (physical function, self-esteem, sexual life, public distress, and work) with the AspireAssist group showing a greater increase in total Impact of Weight on Quality of Life score than the Lifestyle Counseling group (p=0.03).
Mean plasma electrolytes (potassium, sodium, chloride, carbon dioxide), calcium and magnesium concentrations at 52 weeks of therapy were not different than those at baseline in both the AspireAssist and Lifestyle Counseling groups. However, hypokalemia (ranging from 3.2 to 3.7 mEq/l) was reported as an adverse event by the study site investigators in four participants, and was successfully treated with oral potassium supplementation.
At week 52 of therapy, no participant in the AspireAssist group developed hip or whole-body T-Scores of ≤−2.5 s.d. below the normal peak values. The hip T-score decreased slightly (1.08±1.15 at baseline to 0.97±1.19 at 52 weeks, p=0.1) in AspireAssist subjects, consistent with the expected reduction in bone mineral density that occurs with weight loss. Spine bone mineral density in AspireAssist participants was greater at the week 52 than at baseline, an artifact of the Skin Port sitting directly over the spine area being evaluated and influencing the reading.
Approximately 90% of the study-related adverse events (SAEs) in the AspireAssist group were those known to be associated with percutaneous endoscopic gastrostomy tubes, and approximately half of all SAEs occurred within the first seven days after A-tube placement. The development of peristomal granulation tissue occurred later, at one–two months after A-tube placement. Four A-Tubes were replaced because of A-Tube deterioration in the study: three because of fungal growth and one because of punctures of an unknown origin. No A-Tube had to be removed because of clogs. The investigators explained that the durability of the AspireAssist A-Tube appears to be significantly greater than PEG Tubes - with less than 5% of AspireAssist subjects requiring A-tube replacements in the first year - possible reasons for this greater durability are its low profile and larger diameter.
“In conclusion, the use of AspireAssist causes considerable weight loss and is more effective than intensive lifestyle modification alone in the treatment of obesity. The system is designed for the long-term treatment of obesity and necessitates regular monitoring, both aspects of which are important for treatment of a chronic disease<” the authors write. “The placement procedure is the same as that used for percutaneous endoscopic gastrostomy tube placement and can be performed in an outpatient setting. It can also be removed if it is later decided to discontinue therapy, and does not cause anatomical changes that would preclude future bariatric surgery. The weight loss efficacy and safety profile of AspireAssist suggest this treatment approach may help bridge the therapeutic gap between more conservative lifestyle modification and the established bariatric surgical procedures for people with Class II and Class III obesity.”
“Patients with AspireAssist therapy have the opportunity not only to lose a significant amount of weight in a safe and controlled manner, but also to develop a healthier life style through more mindful eating habits,” said Louis Aronne, MD, FACP, the Sanford I. Weill Professor of Metabolic Research at Weill-Cornell Medicine and a co-Principal Investigator of the PATHWAY study.
“With less than 1% of the 29 million Americans with BMIs over 35 availing themselves of bariatric surgery each year, there is a significant unmet need for a non-surgical weight loss procedure that is effective, safe, and reversible. AspireAssist therapy satisfies this need and additionally offers a lower cost solution to the healthcare system. Publication of the PATHWAY results should help facilitate insurance coverage,” said Katherine Crothall, PhD, President & CEO of Aspire Bariatrics.